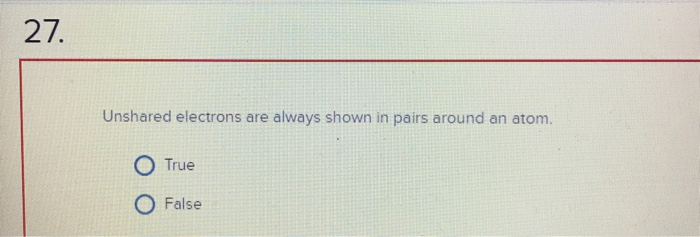
Unshared Electrons Are Always Shown in Pairs Around an Atom.
The sulfur atom can expand its octet of electrons because it has ___. Around the atom to which they belong.
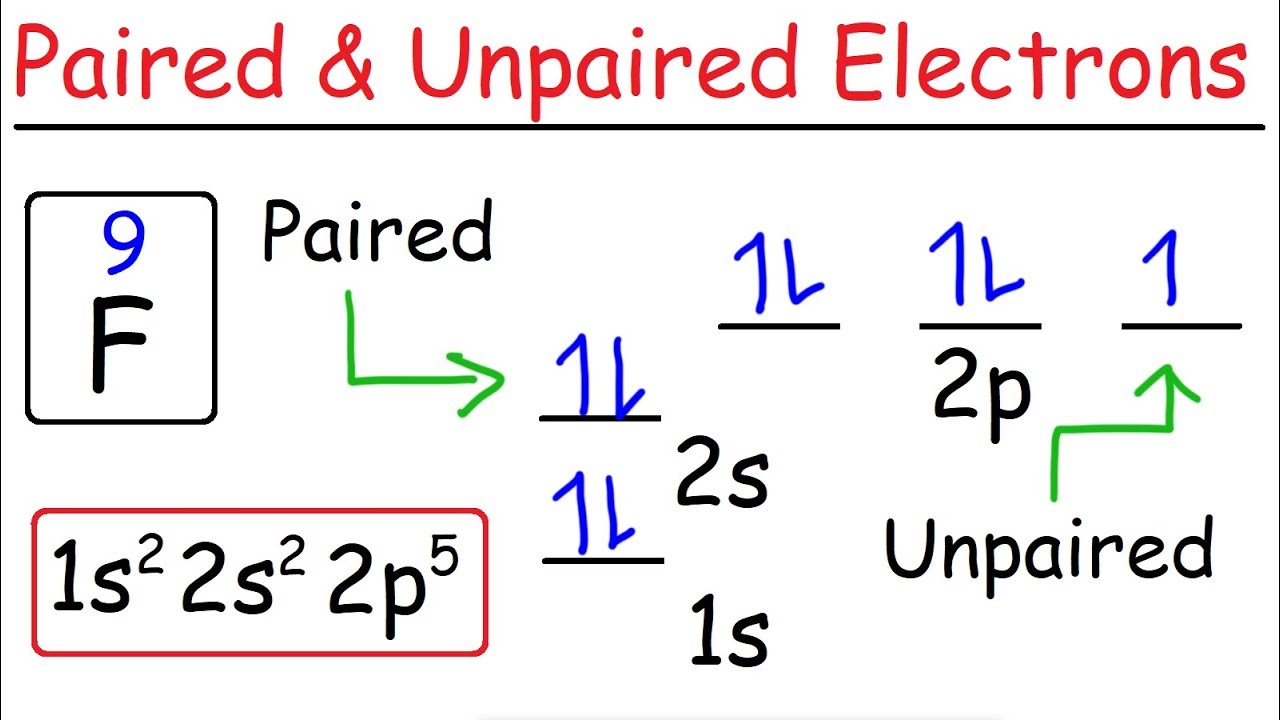
How To Determine The Number Of Paired And Unpaired Electrons Youtube
Calculate the formal charge of each element.
. Which of these species make an isoelectronic pair. A n ________ is a representation of covalent bonding in which shared electron pairs are shown either as dashes or as pairs of dots between two atoms and unshared electrons are shown as dots around the individual atoms. These are shown as dots.
Formal charge on beryllium atom of BeI2 molecule 2- 0-. The beryllium core atom two single bonds connected to iodines of the BeI2 molecule has two valence electrons zero lone pair electrons and four bonding electrons. After placing the lone pairs around the terminal atoms and any remaining around the central atom in a lewis structure if the central atom is not surrounded by 4 electron pairs you must.
Previous question Next question. The bonds produced between the atoms are written as straight lines. No unshared pairs in CH4 so no need to worry about this C H H H H 4.
D Theories of bonding have little relationship to experimental observation of molecular shapes and properties. Unshared electrons are always shown in pairs around an atom. These two remaining VE form a lone pair 2 VEs x lone pair2 VE 1 LP.
The most prohibitive repulsion is lone pair lone pair followed in order by lone pair bond pair and bond pair bond pair. Pairs of valence electrons. Unshared electrons are always shown in pairs around an atom.
Draw the Lewis dot structure for how many total valence electrons for this molecule. Valence electrons of Iodine 7. Experts are tested by Chegg as specialists in their subject area.
We review their content and use your feedback to keep the quality high. A The four bonds in the CH 4 methane molecule are equivalent. Sulfur is the central atom as less electronegative in nature.
Formal charge Group number number of unshared electrons number of electron-pair bonds In BeI2 both peripheral atoms Iodine have the same number of bonding electrons and lone pair electrons so calculate the formal charge for any Iodine atom. According to VSEPR molecules adjust their shapes to keep which of the following as far away as possible. Now how many did it end up with.
The formal charge on Iodine atom. E-s element ended up with unshared e-s 1 2 bonding electrons So for C FC 4 - 0 1. Identify the INCORRECT statement below.
Unshared electrons are always shown in pairs around an atom. Just enter the numeric value 2. As the electrons from the nitrogen lone pair move towards the neighboring carbon to make a new pi bond the pi electrons making up the CO bond must be displaced towards the oxygen to avoid ending up with.
Three atoms around a central atom with no unshared pairs of electrons gives a flat triangular shape a trigonal planar arrangement with all of the bonds forming angles of 120Note that there are four bonds a double bond and two single bonds around the S-atom in sulfur trioxide but only three thangs. For the structure on the left the formal charges will be. In the figure below the electrons belonging to carbon are shown in red and the electrons belonging to oxygen are shown in blue.
Unshared electrons are always shown in pairs around an atom. O True O False. Formal Charge e-s element started with - e-s element ended up with Carbon started with 4 es.
The electron pairs around a central atom will adopt a spatial ar- rangement that minimizes electron-pair repulsion. In ammonia there are 3 atoms and 1 unshared pair of electrons around the. Based on your Lewis Dot structure for how many PAIRS of shared electrons does the S atom have.
SO 2 must have one lone pair on the central atom. Cl- and Ca 2. 2 count all e- groups around central atom A 3 note positions of love parts and multiple bonds.
The number of electrons assigned by a Lewis Structure is the number of lone pair electrons unshared plus one-half of the shared bonding electrons. Cl- O2- F Ca2 Fe 3. Unshared electrons are always shown in pairs around an atom.
Chemistry questions and answers. 18 VEs -16 VE for bonding leaves two VEs leftover. So Sulfur atom shares electrons with Bromine atom and produces SBr2 molecule.
There are three pairs of unshared electrons on each Bromine atom. 4 count bonding and nonbonding e-. 1 place lowest EN atom in center total valence e- place single bonds first distribute 8 e- to all atoms add multiple bonds as needed.
Next Post Next Ionic compounds tend to form between metals and nonmetals when electrons are transferred from an element with high ionization energy. C Unshared pairs of electrons on the central atom of molecules are relevant to the properties of molecules. 2 has 18 valence electrons and 2 bonds around the central atom which require 2 bonds x 8 VEatom 16 VEs.
Convert one or two of the lone pairs on a terminal atom to a double or triple bond between the central atom and terminal atoms. Two bonding atoms and one lone pair yield a bent geometry. The electron distribution when a central atom is surrounded by more than four electron pairs true Unshared lone electrons are always shown in pairs around an atom.
Among several structures involving 900 interactions the most. Asked Aug 24 2019 in Chemistry by FabKid. Put these values for the beryllium atom in the formula above.
A Unshared electron pairs lone pairs located on a given atom can only move to an adjacent position to make a new pi bond to the next atom. Of covalent bonding in which shared electron pairs are shown either as dashes or as pairs of dots between two atoms and unshared electrons are shown as dots around the individual atoms. Likewise SO 2 Fig.
It has four unshared electrons. A series that has 2 or more species that have identical electron configurations but different nuclear charges.

Solved 27 Unshared Electrons Are Always Shown In Pairs Chegg Com
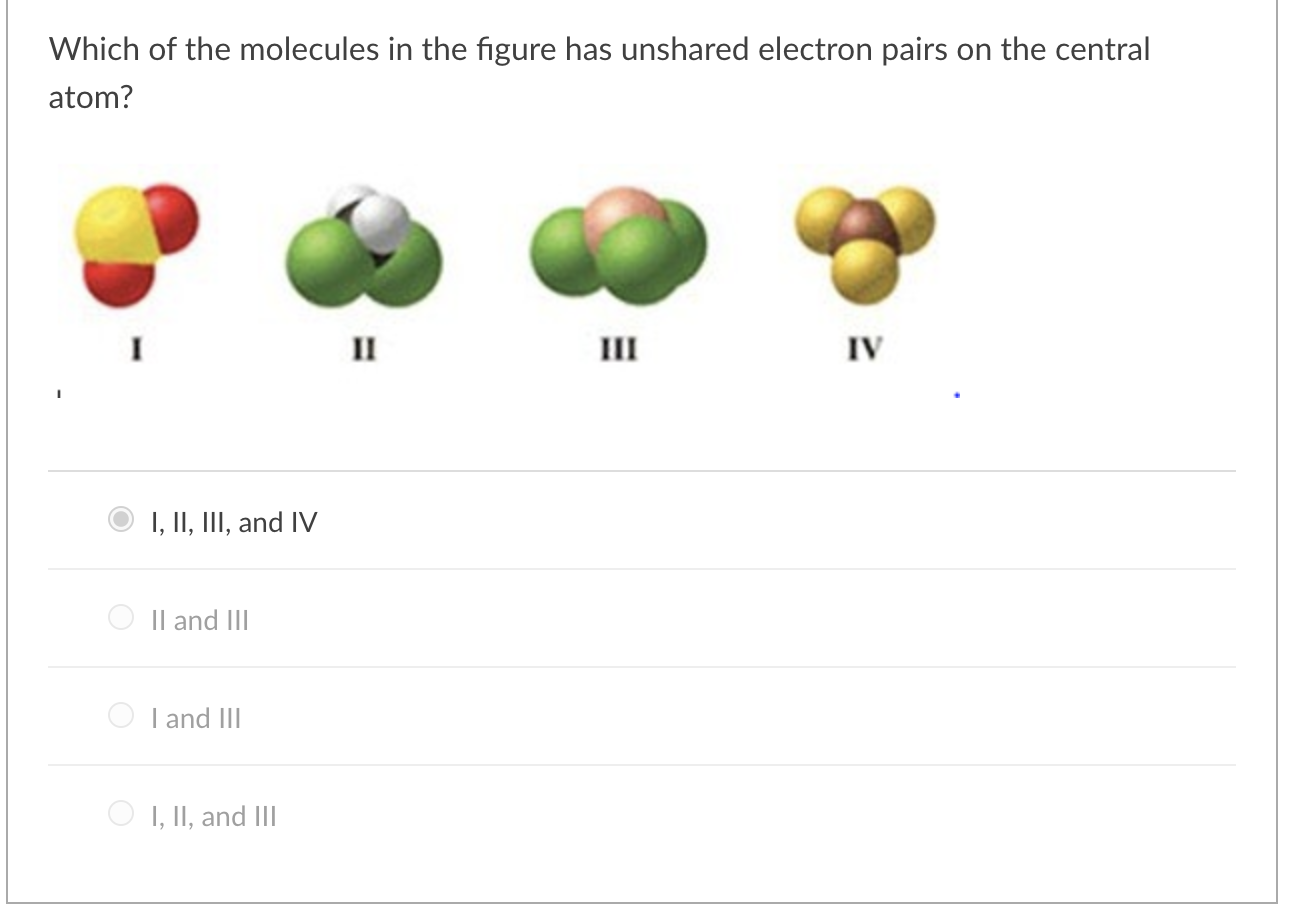
Solved Which Of The Molecules In The Figure Has Unshared Chegg Com
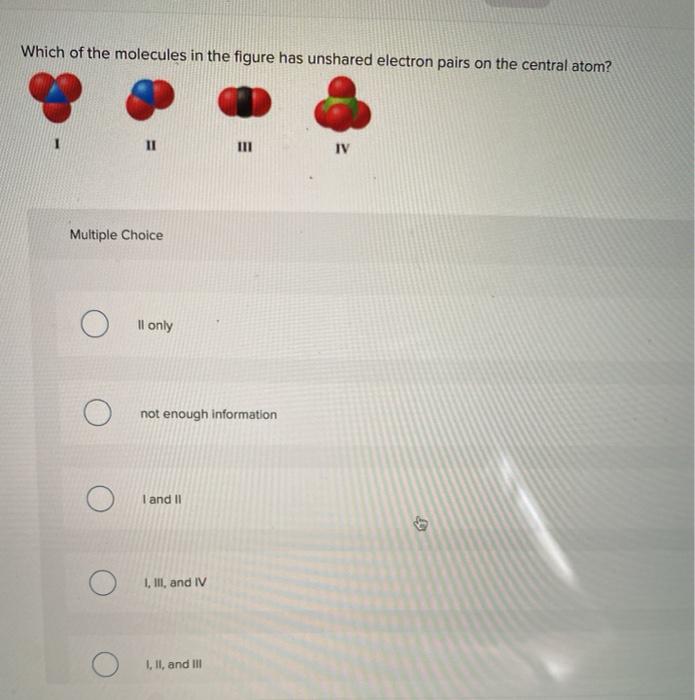
Solved Which Of The Molecules In The Figure Has Unshared Chegg Com
No comments for "Unshared Electrons Are Always Shown in Pairs Around an Atom."
Post a Comment